|
7- Photoreduction Of Thionine By Monomethylamine In 50%
Aqueous Methanol
Fahim Uddin*,
Qazi Zaheerul Hasnain and Rehana Saeed
Department of Chemistry, University of Karachi, Karachi- 75270
Pakistan. email:
fahim_uddin01@yahoo.com
Abstract
The photochemical reaction of thionine [Th] with monomethylamine was
studied in 50% aqueous methanol at 25oC ± 0.1 in a
specially constructed apparatus. The acidities of the reaction
solutions were determined by using Hammett acidity function [Ho].
The effect of concentration of monomethylamine, thionine, acidity and
temperature on quantum yield (f) was studied and found that quantum yield is a
function of acidity as well as concentration of reductant [AH2]
and temperature. The results interpreted in terms of proposed reaction
mechanism led to the conclusion that quantum yield of the reactions of
thionine with monomethylamine is controlled by two equilibria between
i) triplet state of thionine [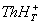 ] with proton and protonated triplet state of
thionine [ ] with proton and protonated triplet state of
thionine [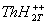 ] and ii) protonated triplet state of thionine with
monomethylamine and associated complex i.e. [ThH2T++.AH2] ] and ii) protonated triplet state of thionine with
monomethylamine and associated complex i.e. [ThH2T++.AH2]
<<< |

