|
8.
Quantum Yield of Photoreduction of Methylene Green with
Trimethylamine
Rehana
Saeed and Fahim Uddin*
Photochem
Research Lab, Dept. of Chemistry, University of Karachi,
Karachi-75270 Pakistan
*To whom all correspondence. email:
fahim_uddin01@yahoo.com
Abstract:
The kinetics of photo exicited triplet methylene green with
trimethylamine in 50% aqueous isopropanol was studied at
different temperatures. The kinetic data was analysed in terms
of quantum yield (j) for the photochemical reduction of
methylene green with trimethylamine was determined as a function
of concentrations of methylene green, concentrations of
reductant [AH2], acidity [Ho] and
temperature. The photochemical reduction was carried out in a
special type of optical processor. The optical processor has a
monochromatic arrangement, photocells, calibrated galvanometer,
a double walled reaction cell associated with a deoxygenating
system, a temperature controlling unit and a magnetic stirring
system. A monochromatic light of 657 nm wavelength was used for
irradiation of oxygen free reaction solutions and transmitted
light was measured and recorded in terms of electrical signals.
It was observed that the quantum yield varies with reductant
concentration [AH2] and is independent of the
concentration of methylene green at specific values of acidity
and temperature. The values of specific rate constants and
ratios of rate constants were determined. The results have been
interpreted in terms of the reaction mechanism showed the
existence of two equilibria: i.e. one between the triplet state
of methylene green with the proton and the protonated triplet
state of methylene green [ ],
and the other between the protonated triplet state of methylene
green with reductant and associated the complex of methylene
green [ ],
and the other between the protonated triplet state of methylene
green with reductant and associated the complex of methylene
green [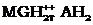 ].
Thermodynamic parameters were also evaluated as a function of
acidity. ].
Thermodynamic parameters were also evaluated as a function of
acidity.
Key words: Photokinetics, methylene green, trimethylamine,
quantum yield, mechanism, thermodynamic parameters.
<<< |

