|
9.
First and novel mechanistic studies of Os (VIII) catalysis in
oxidation of dimethyl sulphoxide by alkaline Br (V): A kinetic study
Bharat Singh*, Aniruddh Kumar Singh, Kumud Lata Singh, R. Dubey and
S.K.Singh
Chemical kinetics laboratories, Department of chemistry, University
of Allahabad,
Allahabad-211002, India
Abstract:
Kinetics of osmium (VIII) catalysis in oxidation of dimethyl
sulphoxide (DMSO) by alkaline solution of potassium bromate
have been studied in the temperature range of 300C to
450C using mercuric acetate as Br - ions scavenger.
The reaction is first –order with respect to Os (VIII) and [KBrO3]
.The order in DMSO decreases from unity at its low
concentrations to zero at high DMSO concentrations. Accelerating
effect of [OH-] on the rate of osmium (VIII) catalysed oxidation
of DMSO by Br (V) has been observed while negligible effect of
variation of both [Hg (OAc)2] and ionic strength of
the medium has been found. Formation of most reactive activated
complex (A) prior to attack by oxidising species of Br (V) in
the slow and rate
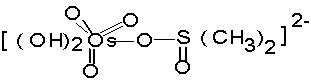
(A)
determining step is
proposed. Various activation parameters have been computed and a
suitable mechanism conforming to the observed kinetic data is
proposed and discussed.
KEY WORDS: Mechanism, Oxidation kinetics, DMSO, Os (VIII)
catalysis, Br (V)
<<< |

