|
8.
Kinetic and Mechanistic Study of Hg (II) Co-Catalysed and RuO4
Catalysed Br (V) Oxidation of Erythritol and Dulcitol in Alkaline
Medium
Bharat
Singh*,Aniruddh Kumar Singh, Chhaya Singh, Ashish and Kumud Lata
Singh
Department of Chemistry, University of Allahabad, Allahabad-211002
(India)
E-mail:
singh.aniruddh02@gmail.com;
Tel +
91532-2500553
Abstract.
The kinetics of Hg (II) co-catalysed and RuO4 catalysed
oxidation of erythritol (Ery) and dulcitol (Dul) by potassium
bromate in alkaline solution exhibit zero order in potassium
bromate and first-order in both [RuO4] and [OH-]. First-order
kinetics in low [substrates] tends to zero-order at their higher
concentrations. Although mercuric acetate has been used as Br-
ions scavenger but variation of its concentration shows positive
effect on reaction rates. Addition of NaClO4 decreased the
reaction rate, showing negative effect of ionic strength of the
medium. Mechanistic steps, supported by activation parameters
and conforming to the observed kinetics, are discussed.
Graphical Abstract. Mechanistic steps based on kinetic
studies of Hg (II) co-catalysed and RuO4 catalysed oxidation of
erythritol and dulcitol by alkaline solution of potassium
bromate have been reported and erythrionic acid and galactonic
acid respectively have been detected as reaction products. The
following rate law derived on the basis of reaction scheme
conforms to all the kinetic observations, stoichiometry and
reaction products.
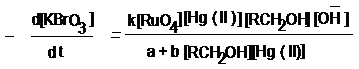
Where k = 2/3 kd k1 k2, a
= k-1 k-2 and b = kd k2
KEY WORDS:
Oxidation, kinetics, KBrO3, Ru(VIII)catalysis,
erythritol, dulcitol.
<<< |

