|
10.
Mechanistic aspect of osmium (VIII) catalysed oxidation of crotonic
acid
by aqueous
alkaline solution of chloramine-T: A kinetic modeling
Bharat Singh*, Aniruddh Kumar Singh, Anamika Singh & Shipra Tripathi
Chemical Kinetics Laboratory, Department of Chemistry, University of
Allahabad, Allahabad 211 002, India
Email:
bschem_au@yahoo.com
Abstract:
Kinetics and mechanism of Os(VIII) catalysed oxidation of
crotonic acid (CA) by alkaline solution of chloramine-T have
been investigated at 35°C. First order kinetics with respect to
each chloramine-T and Os(VIII) was observed. The reaction
exhibits linear dependence of reaction rate at low [CA] tending
towards zero order at its higher concentrations. Accelerating
effect of [OH Ż ] ion on reaction rate was observed. The
reaction shows positive effect of variation of ionic strength of
medium while successive addition of para – toluenesulphonamide
(a reduction product of chloramine-T) decreases the rate. ClOŻ
and [OsO4(OH)2]-2 have been
postulated as the reactive species of chloramine-T and Os(VIII),
respectively. The reaction was studied at four different
temperatures and the overall activation parameters have been
computed. The main oxidation products have been identified as
acetaldehyde and glyoxylic acid. A suitable mechanism involving
attack of chloramine-T species on complex formed between
catalytic species of osmium tetroxide and crotonic acid in the
slow and rate determining step is suggested and the following
rate law consistent with observed kinetic results is presented.
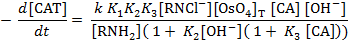
Keywords: Kinetics, Reaction mechanisms, Chloramine-T, Crotonic
acid, Osmium tetroxide
<<< |

