|
4.
Mechanistic studies of aquachlororhodium(III) catalysed oxidation of
D-threonine by chloramine-T in acidic medium: A kinetic modeling
Bharat Singh*, Aniruddh Kumar Singh, Anamika Singh &
Shipra Tripathi
Chemical Kinetic Laboratory, Department of Chemistry, University of
Allahabad, Allahabad 211 002, India
Email:
bschem_au@yahoo.com
Abstract:
The kinetics of homogeneously Rh(III)
catalysed oxidation of D-threonine by chloramine-T in the
presence of perchloric acid has been investigated at 308
K.The reaction follows complex kinetics, being first order
in [chloramine-T], zero order in D-threonine and inverse
fractional order in both [H+] and [Cl¯].The rate
of the reaction shows first order dependence on [Rh(III)].
Variation of ionic strength of the medium and addition of
various amounts of the reaction product, p-toluenesulphonamide
or ClO4¯ ions had no effect on the rate. RNHCl
and [RhCl4(H2O)2]-1
have been postulated as the reactive species of chloramine-T
and Rh(III), respectively. Activation parameters for the
slow and rate determining step of the proposed mechanism
which involves the formation of the most activated complex
(C4), [Cl3(H2O)2Rh(RNHCl)]
formed in the slowest step and prior to its attack on the
substrate molecule, threonine have been obtained from rate
measurements at 30, 35, 40 and 45°C. The rate law in
conformity with the observed kinetic data has been derived
as
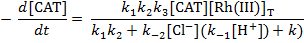
The main oxidation product has been
identified as 2-hydroxypropanenitrile.
Keywords: Kinetics, mechanism, Oxidation, D-threonine,
chloramine-T, Rh(III)chloride
<<< |

